|
1. Water and its hydrogen bonding (Figures 1 & 2). Atoms are the building blocks of materials in the Earth and on its surface, and are composed of two fundamental items: a nucleus, which is positively charged, and electrons, which are negatively charged and orbit around the nucleus (the orbits are called shells). The nucleus may be composed of only positively charged protons, but may also contain neutral-charged neutrons, see Figure 1A & B. The number of protons equals the number of electrons so that the entire atom has no charge, examples are hydrogen and oxygen, see Figure 1C.
Atoms react with other atoms in order to become more stable, forming compounds. For water, this process has unusual consequences. A single atom of oxygen has two electron shells; the first is filled by two electrons, the second contains only six, but needs 8 to be filled and stabilised. >> |
A single atom of oxygen has two electron shells; the first is filled by two electrons, the second contains only six, but needs 8 to be filled and stabilised. Therefore, it requires 2 extra electrons, and it gains these by reacting with an atom, or atoms, which can lose electrons (Figure 1D). Hydrogen has a tendency to lose its single electron, so two hydrogen atoms will react with one oxygen atom and chemically bond together in a covalent bond (where electrons are shared to satisfy the need for electrical neutrality in the molecule). In the case of water, this covalent bonding between oxygen and hydrogen is slightly unequal. The oxygen atom is more electronegative (it is better than hydrogen at attracting electrons, because it has more positively charged protons in its nucleus), and this makes it slightly more negative; consequently, the hydrogen atoms are unable to hold the electrons near to them, and become slightly more positive. |
|
The imbalance of attraction between hydrogen and oxygen for electrons leads to asymmetry in the shape of water molecules. For reasons that do not need to concern us, electrons in the shells of oxygen are arranged in pairs. Because each pair of electrons in the molecule has the same negative charge, pairs repel other pairs, and the shape which allows the electron pairs to be as far apart as possible is a tetrahedron (Figure 1D). The hydrogen ions come to sit at any 2 of the 4 corners of the tetrahedron, and therefore always lie near each other at one side of the molecule. The two pairs of unshared electrons at the oxygen side of the molecule, combined with the higher electronegativity of the oxygen atom, produce a slight negative charge at the oxygen end. >> |
Because oxygen is several times larger than hydrogen, the whole water molecule behaves as a sphere with two small lumps near each other on one side. The molecule therefore develops an overall polarity of charge, called a dipole. So, in contrast to most other liquids (which are usually a collection of freely-moving molecules), in water the dipoles interact and weakly bond together, where the negatively-charged end of a water molecule attracts the positive end of another water molecule. This weak attractive force is termed hydrogen bonding (Figure 2); without it water would be a gas, not a liquid, on the Earth surface, so oceans would not exist. Also either the biosphere would not exist, or it would have a very different nature from its present arrangement. |
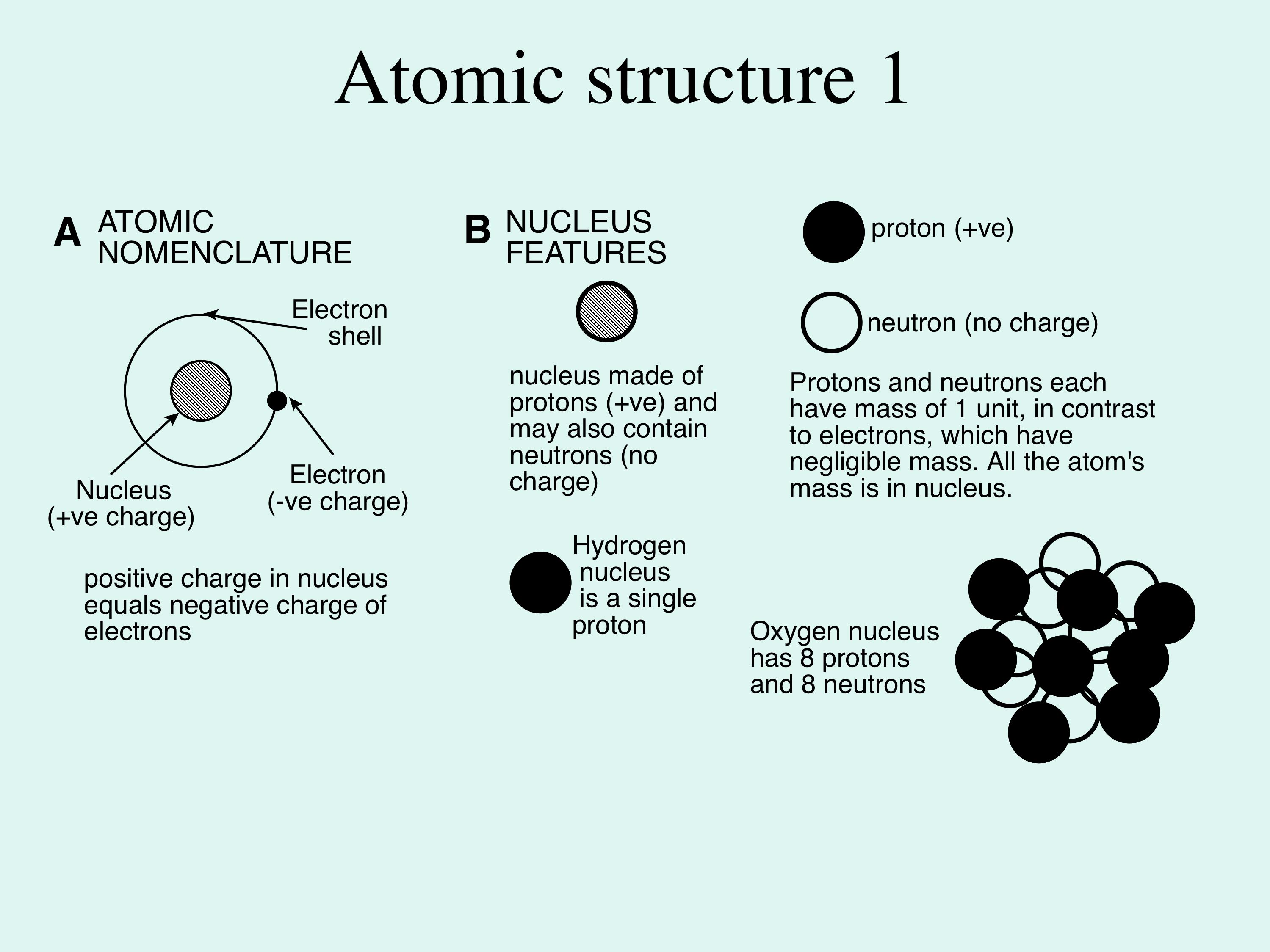
Figure 1A and B; these show the basic idea of the structure and make-up of atoms; please see text for explanation.
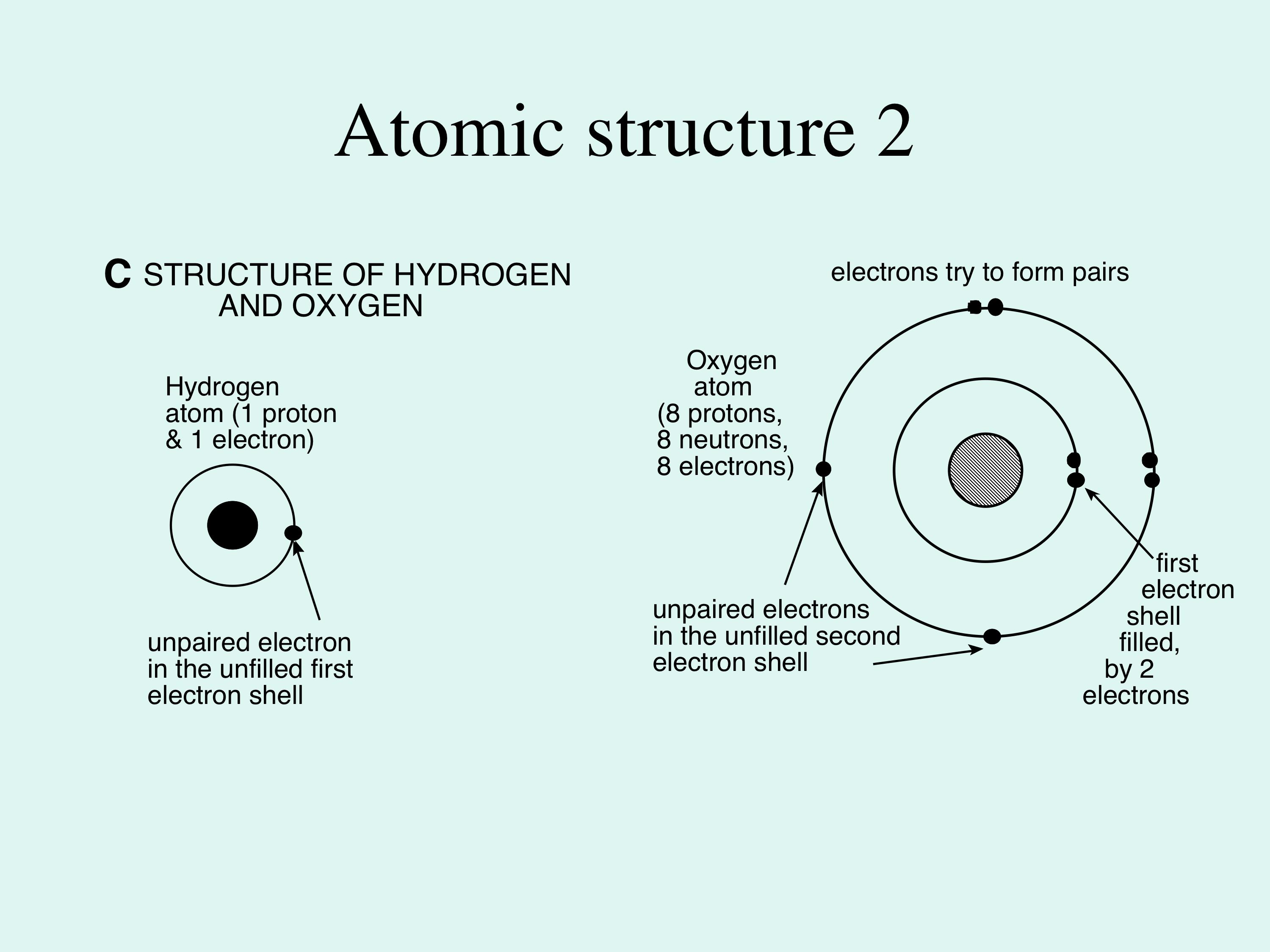
Figure 1C: this shows the atomic structure of hydrogen and oxygen; in each case the nucleus consists of the essential protons, one for hydrogen and 8 for oxygen - this number defines the chemical elements. In oxygen there is additional mass provided by neutrons. In both atoms the positive electrical charge of the proton is balanced by equal numbers of negative charges in the orbiting electrons, shown here as concentric circles for simplicity (the electron orbits are actually shells, so the electron moves in 3-dimensions around the nucleus).
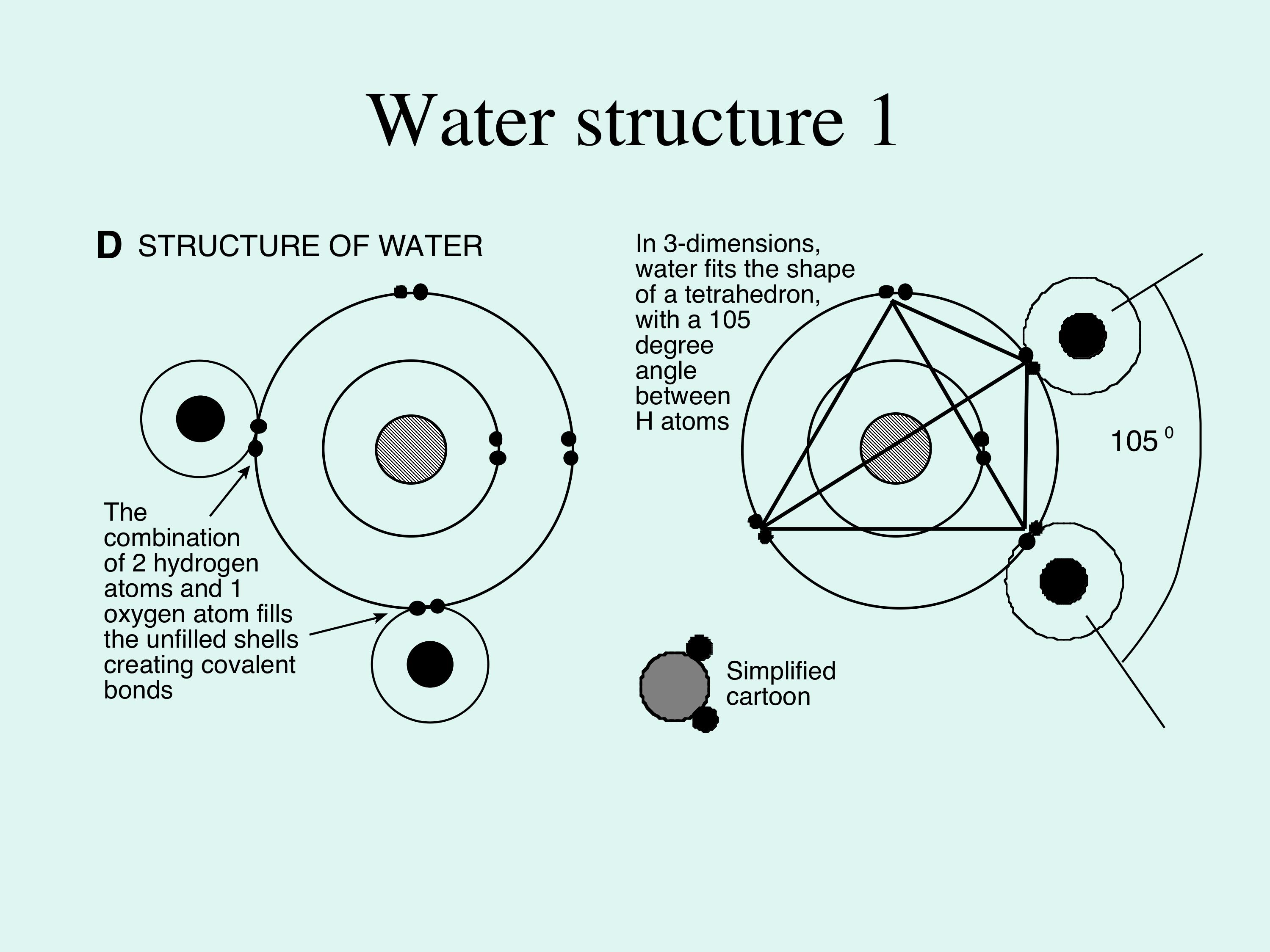
Figure 1D; this shows the molecular structure of water; notice how the two hydrogens are close together on one side of the oxygen - this is the reason why that side of the water molecule is slightly positively charged in contrast to the slight negative charge on the other side. This charge difference induces a polarity to the molecule, which attracts other water molecules to form hydrogen bonding.
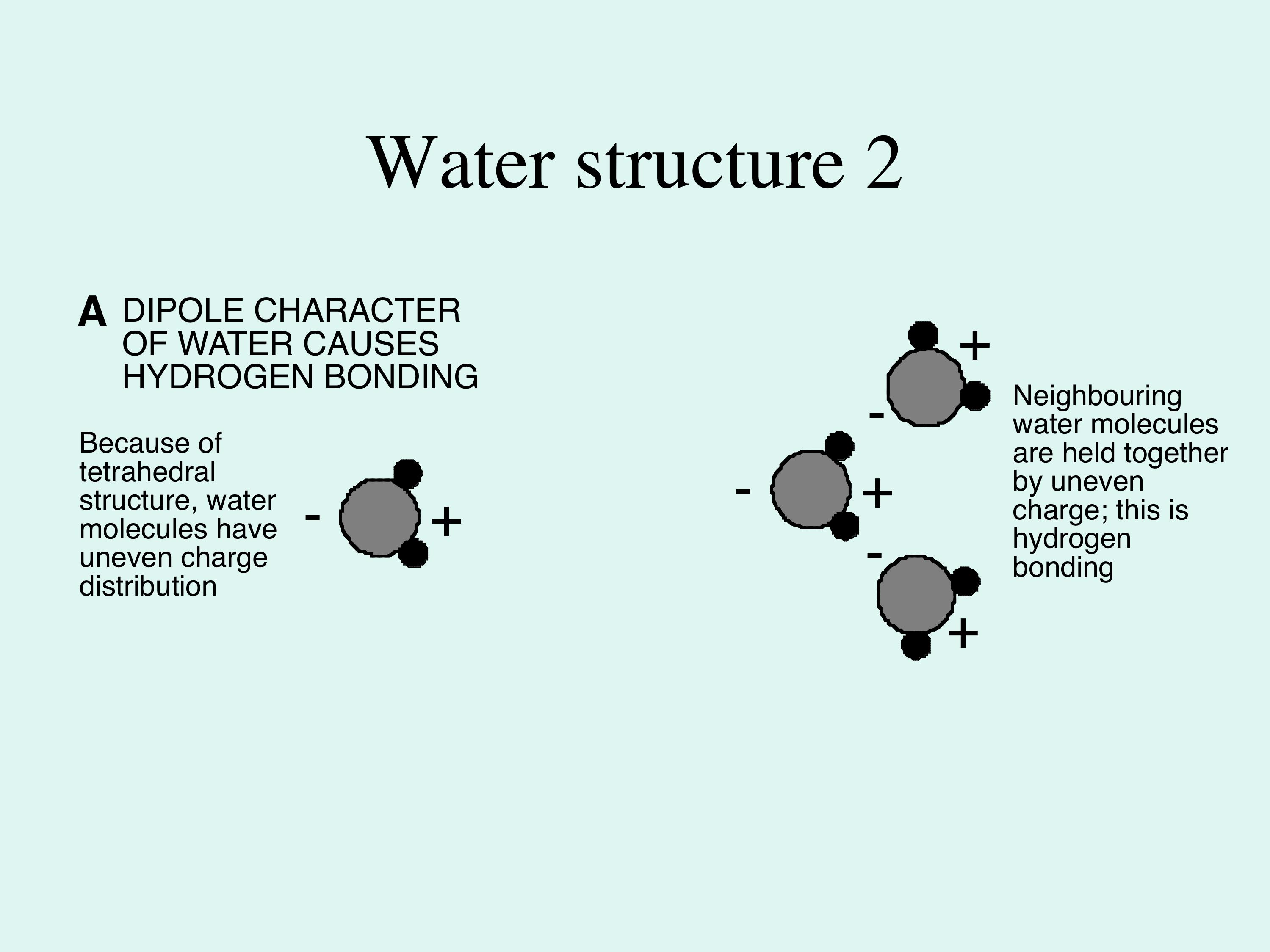
Figure 2A; a simpler version compared to Figure 1D; it shows the water molecules in simple form, and emphasises the charge distribution across the molecule.
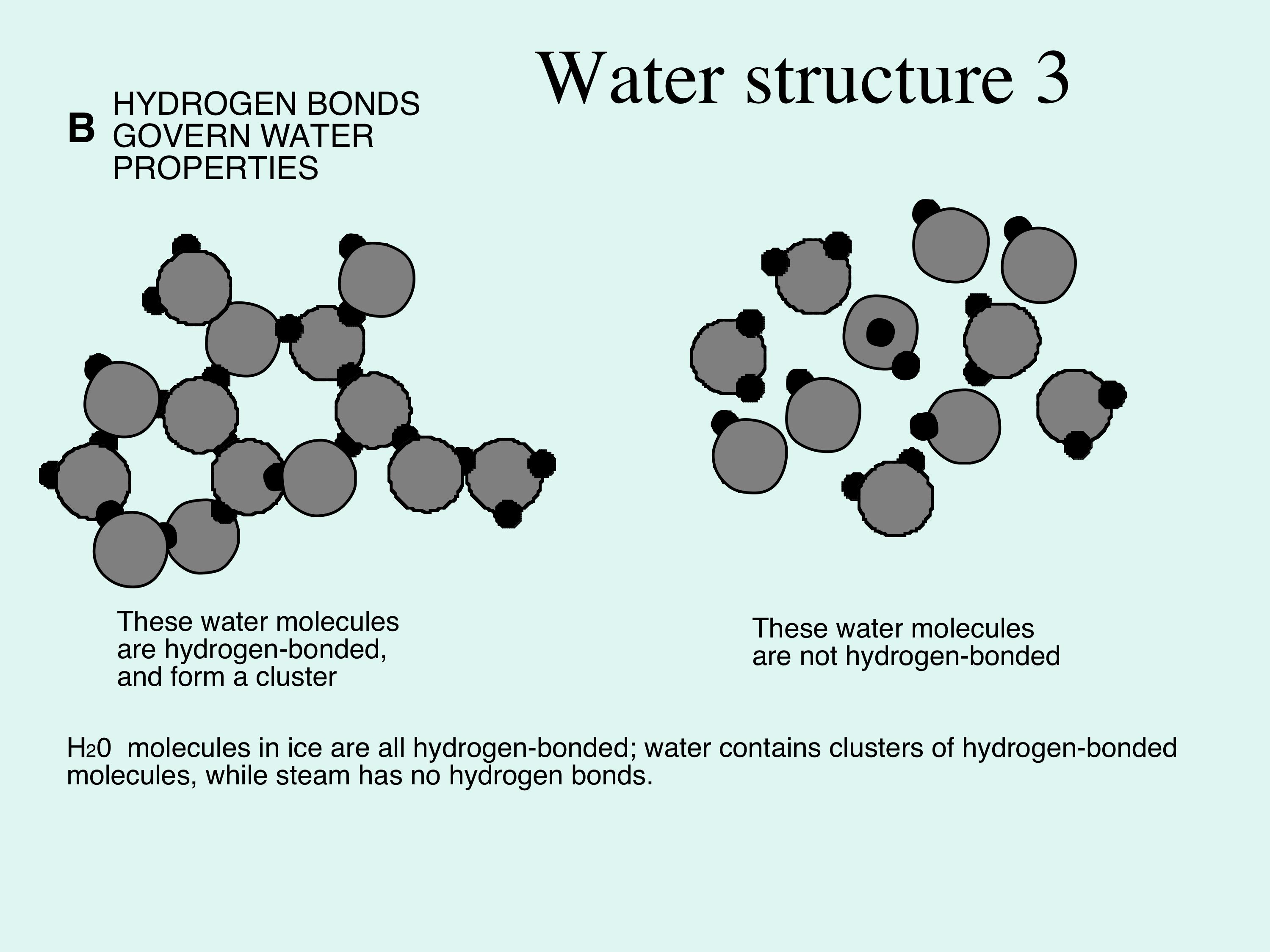
Figure 2B; hydrogen bonding in action. On the left, all the water molecules are held together, this is ice, but the diagram also represents water because in water only some of the hydrogen bonds are broken, so there are clusters of water molecules still held together by hydrogen bonds, as shown here. On the right, all the hydrogen bonds are broken, the water molecules are separate, this is water vapour, which can also be steam.
|
2. Why is hydrogen bonding important? (Figure 3). Figure 3A & B shows two examples of why hydrogen bonding is important. Figure 3A shows the temperature range of the three states of water (solid, liquid, gas/vapour) in comparison with its nearest chemical compound, hydrogen sulphide. You can see that hydrogen sulphide exists as only a gas at normal surface temperatures, and it requires much colder conditions to liquefy and freeze hydrogen sulphide. The reason is that hydrogen sulphide does not have hydrogen bonding that allows the formation of water at Earth-surface temperatures (average of 15 degrees centigrade). Figure 3B is a graph that shows what happens when water changes its state between solid/liquid and liquid/gas; starting with ice at the bottom left of the graph, moving from left to right along the graph, ice requires heat to break enough hydrogen bonds to turn it into liquid (water); notice the temperature does not change as the heat is added, up to a certain point. This point is when enough hydrogen bonds are broken so that the ice can no longer stay as ice, and becomes water. >> |
Then adding more heat raises the temperature, as would happen in your kettle when you are making a cup of tea. Then when water is boiling, more heat is required to break all the remaining bonds to turn it into vapour; while that process is taking place, again the temperature does not increase. If temperature is reduced, the process simply reverses; thus the breaking and forming of hydrogen bonds is a reversible process depending on the energy (heat) of the system. Thus these two events of state change (ice/water and water/vapour) simply break/make hydrogen bonds, and the ice/water/vapour change does not change temperature. This heat is therefore hidden in changing the water structure, it is not measurable using a thermometer and is therefore called latent heat. In contrast, the temperature of water as it warms up between 0 to 100 degrees can be measured with a thermometer, it is therefore sensed by the thermometer, and is called sensible heat. |
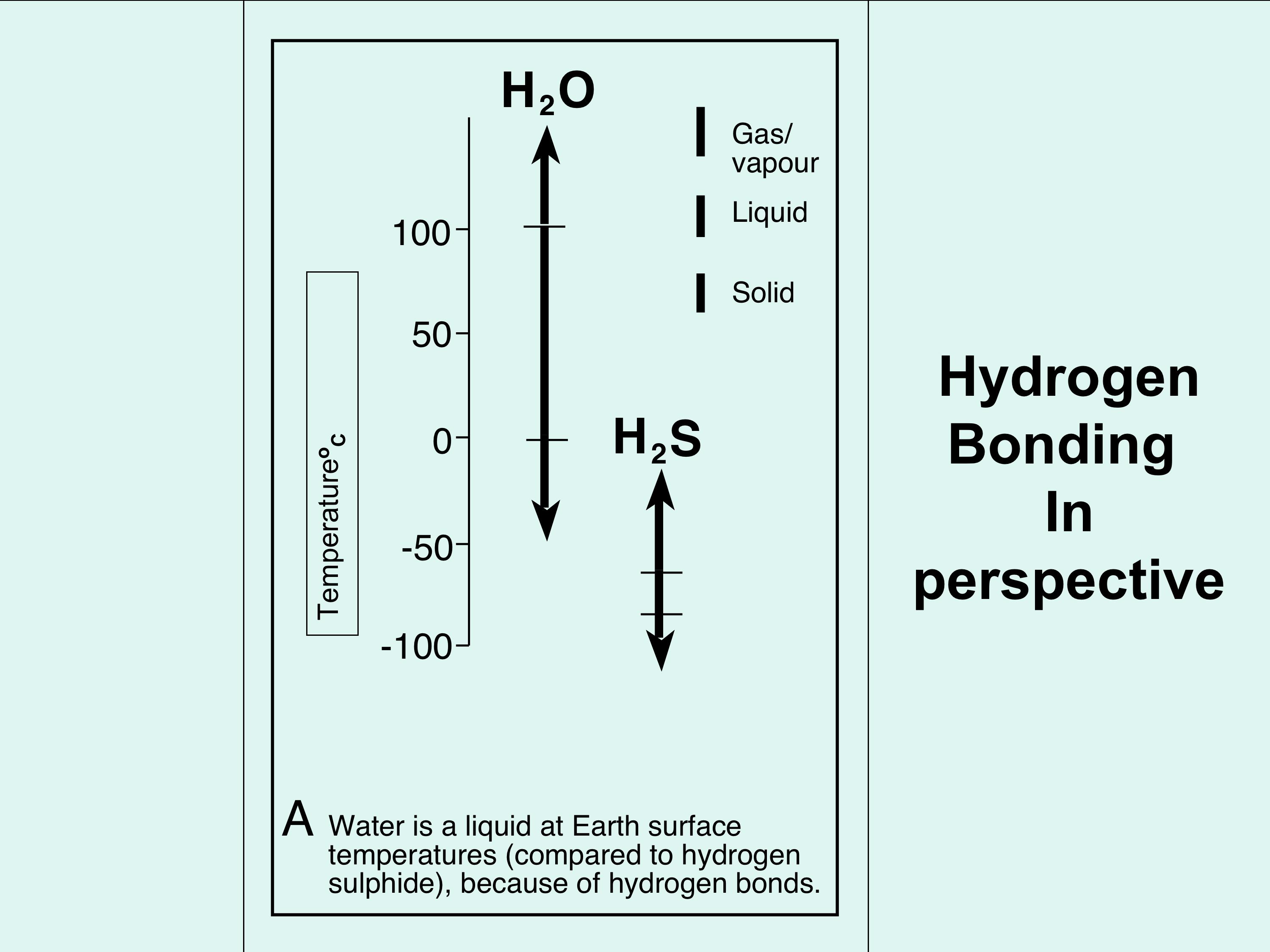
Figure 3A; a simple representation of the different physical states of water in comparison to its nearest chemical compound, hydrogen sulphide. Note that because hydrogen sulphide does not have hydrogen bonding, it is a gas above c.60 degrees Centrigrade, when water at that temperature is of course ice.
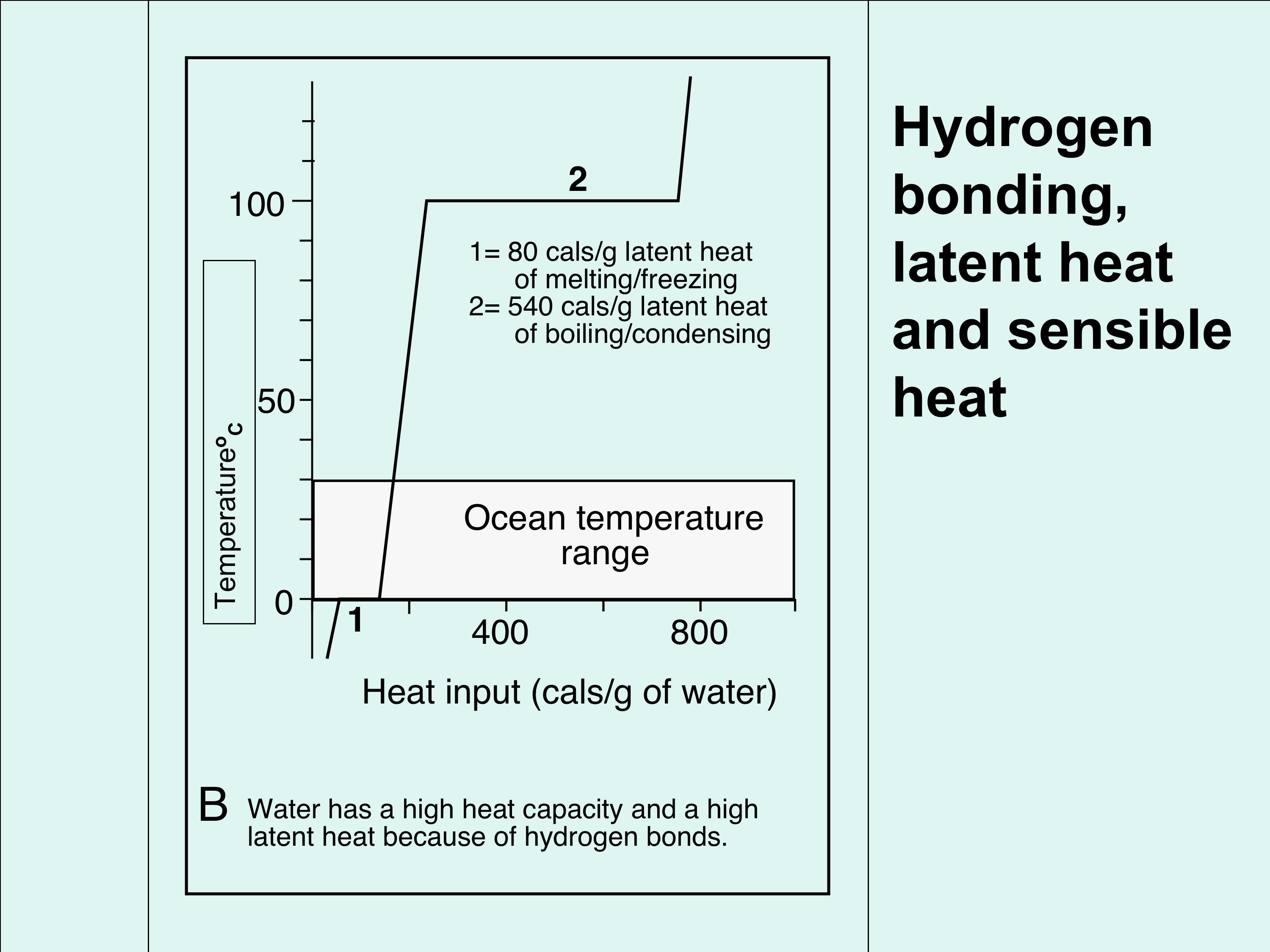
Figure 3B; a simple graph showing the relationship between heat and hydrogen bonding. Heat is required to turn ice into water and water into steam, but this heat does not lead to a change in temperature. It is therefore hidden heat that simply breaks the hydrogen bonds, it is called LATENT HEAT. In contrast, heat that can be measured, between the zones of latent heat, is called SENSIBLE HEAT.
|
3. Dissolution of materials by water (Figure 4) Water is a very effective solvent, and the dissolution capability of water can be illustrated by examining what happens to NaCl (sodium chloride) in contact with water. In the solid state, the Na (sodium) and Cl (chlorine) are held together by ionic bonds, which involves the transfer of electrons, rather than the sharing of electrons characteristic of covalent bonds. Na donates an electron to Cl, by which process both atoms achieve greater stability (Figure 4).
Because a Na atom has lost an electron, it is positively charged (Na+, a cation), whereas each Cl atom, having gained an electron, is negatively charged (Cl-, an anion). >> |
In the solid state, the two are attracted to each other electrostatically, in an ionic bond, which provides the strength to make salt a solid. When immersed in water, the negatively charged end of the H2O molecule takes Na ions from the surface of the solid, while Cl ions are pulled away by the positively charged ends of other water molecules (Figure 4). The water molecules surround the ions, which are now too far apart to exert any attractive force on each other, so the ions may be regarded as dissolved. Because of this surrounding effect, water can dissolve large amounts of material. The process continues until all the salt has dissolved, or the water has become saturated, when it can hold no more ions, and therefore won't dissolve any more. |
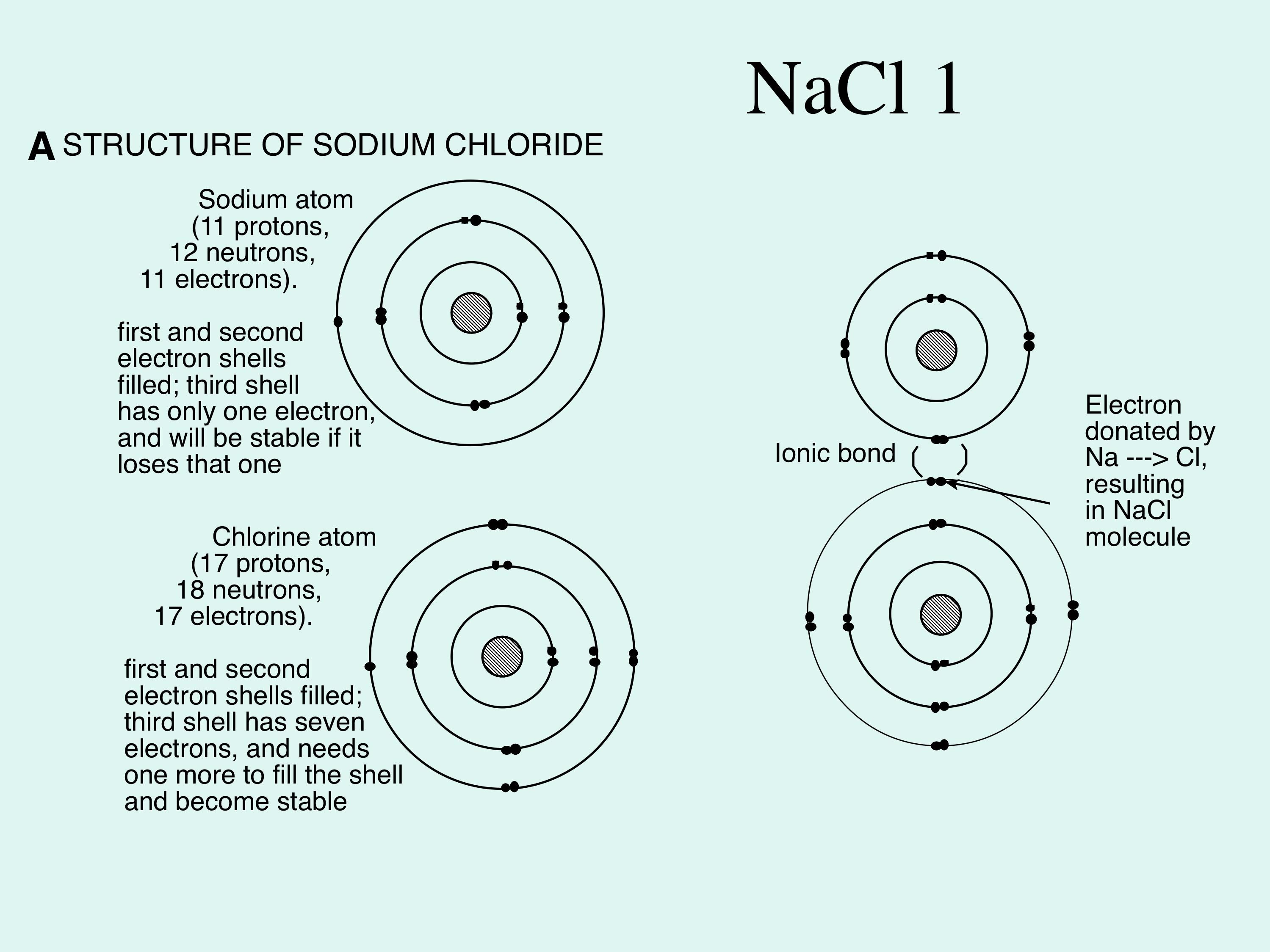
Figure 4A; shows the structure of the most commonly-found material dissolved in water, sodium chloride (salt). Note that electrons are shared between the sodium and chlorine, which is what holds the molecule together as a solid. This is called ionic bonding.
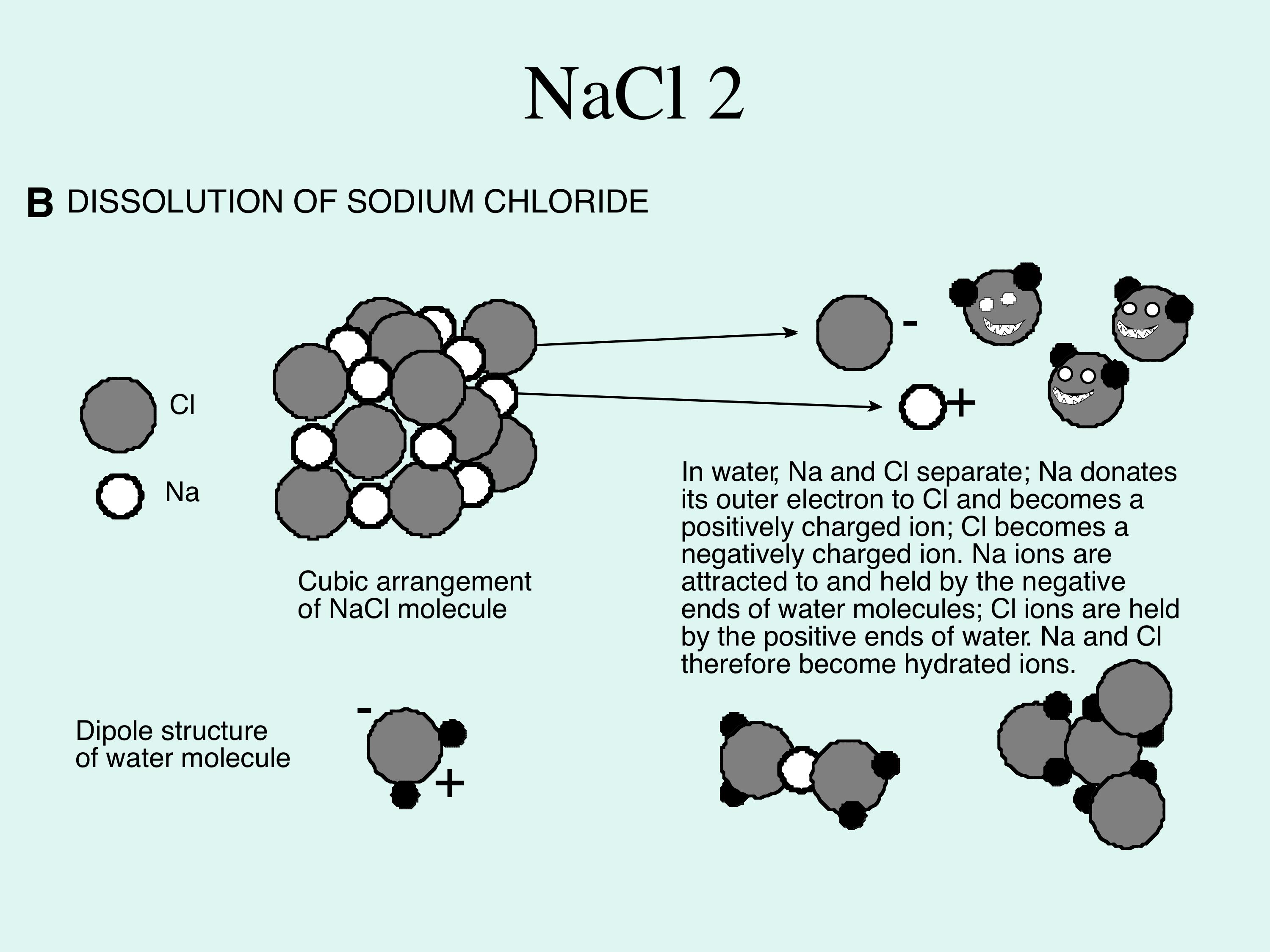
Figure 4B; this shows what happens when salt it dissolved in water; the salt breaks into stable particles called ions. Ions are simply atoms with their electron shells stabilised by addition or loss of electrons, so in the case of salt, the sodium and chlorine can exist separately from each other. The positive charge sodium ions (cations) are attracted to the negative ends of water molecules; the negatively charged chlorine ions are attracted to the positive ends of water molecules. My favourite folk singers, Kate and Anna McGarrigle, wrote a song called NaCl; it has an amusing take on the chemistry of sodium chloride and is worth a listen.
Click here to return to the GENERAL INTEREST PROJECTS header page
Click here to return to the HOMEPAGE