|
Stromatoporoids are calcified sponges that grew on the sea floor, normally using the dead skeletons of other organisms as a hard surface to grow on, as shown in the rather beautiful photo below (Fig. 1) Stromatoporoids were enormously abundant during the 100 million years between the middle Ordovician and Late Devonian Periods, ca. 470 - 370 Ma, so a study of their original mineralogy is of great interest in debates about controlling processes affecting carbonates in the middle part of the Palaeozoic Era. However, identification of their original mineralogy is controversial because there is lack of clarity, and conflicting ideas, about this topic.

Figure 1: Vertical cross section through a dome-shaped layered stromatoporoid (Densastroma pexisum), which grew on an overturned rugose coral of the genus Schlotheimophyllum. Upper Silurian Visby Formation, on the island of Gotland, Sweden.
The identification (taxonomy) of Palaeozoic stromatoporoids is based on the calcareous elements of stromatoporoids; their architecture has been extensively studied by numerous authors, summarized by Stearn (2011). To identify them, stromatoporoids are sectioned vertically and horizontally, and two thin sections are therefore needed for a reliable identification. Moreover, the thin sections need to be around 50-80 microns thick, significantly greater than the normal 30 microns used for thin section work. At 50-80 microns, the structure is very clear, and the samples can look well-preserved. However, if the thin sections are made at normal 30 micron thickness (and better if it is less, down to 15 microns) it becomes clear that even the apparently best-preserved specimens are in fact substantially recrystallised and their structure needs to be understood, which is the aim of this webpage. Thus, simple demonstrations of the physical appearance of stromatoporoids in thin section draw attention to the differences between stromatoporoids and other fossils, described in the rest of this webpage.
In all cases that I have observed, of brachiopods in the same samples and same facies as stromatoporoids, the brachiopod shells are laminated and well-preserved (Fig 2 and 3) in significant contrast to the stromatoporoids, even those stromatoporoids which are considered to be well-preserved. This difference was also reported and illustrated by Rush and Chafetz (1991). Therefore, stromatoporoids were clearly NOT originally low-Mg calcite (LMC).

Figure 3: Vertical thin section of Densastroma pexisum (Upper Silurian Visby Formation, Gotland, Sweden), which was interrupted during growth, so that an atrypid brachiopod was enveloped by the recovered stromatoporoid growth (inset photograph). The main picture is an enlargement showing the well-preserved laminated brachiopod shell contrasting the altered stromatoporoid skeleton. Note that the stromatoporoid shows characteristic irregular elongated crystals arranged normal to the growth surface, overprinting the skeletal structure, discussed in the text and further illustrated in Figs. 7-10. SEM photographs of the contrast between stromatoporoids and brachiopods are given by Rush and Chafetz (1991); unfortunately I do not have any SEM photos of stromatoporoids to include in this webpage.

Figure 4: Cathodoluminescence photo of a brachiopod, Upper Silurian Hemse Group, Gotland, Sweden, showing its well-preserved laminated structure, reflecting the original Lo-Mg calcite mineralogy of brachiopods. This structure is completely different from stromatoporoids, seen in other photos below.
2. ARAGONITE RECRYSTALLISATION AND DISSOLUTION
|
Apparently well-preserved stromatoporoids, showing architectural elements of vertical and horizontal structures, commonly occur in the same beds, even the same samples, as completely recrystallised mollusk shells. Furthermore, repeated observations by the author, of mollusc shells used by stromatoporoids as substrates throughout the Silurian of Gotland and England, demonstrate dissolution of the mollusc shell and collapse of the external mould onto the internal mould, yet the stromatoporoid has not suffered any dissolution (Figs. 5 and 6).

Figure 5: Cross section through a stromatoporoid (S) that grew on the dead shell of an orthoconic nautiloid (O) (left); the nautiloid has been susbstantially dissolved by diagenesis, whereas the stromatoporoid is unaffected. w= outer wall of nautiloid shell, s= siphuncle; septa are also visible as sharp changes in the micrite fill in the nautiloid. A heliolitid coral (C) lies on its side,right, and a recrystallised gastropod (G) is lower right; Visby Formation, Wenlock of Gotland. Both the nautiloid and gastropod have dissolved walls, and the sediment is compacted to fill the space once occupied by their shells, an indication of their original aragonite mineralogy; this is in complete contrast to the stromatoporoid and coral samples in this specimen.

Figure 6A: Thin section (main picture) and polished slab (inset) of Densastroma pexisum, Visby Formation, Wenlock of Gotland. The stromatoporoid grew on a dead mollusc shell that was subsequently dissolved in diagenesis and the sediment collapsed, leaving a thin line to show the location of the shell (blue arrows). The stromatoporoid is unaffected, except that it shows a recrystallised fabric comprising irregular elongate crystals that overprint the skeletal structure; those crystals are normal to the growth surface. The sediment was burrowed (lower centre of photo), revealing unconsolidated sediment. The stromatoporoid used the shell as substrate thereby avoiding the loose sediment; however, other samples, not illustrated in this paper, indicate that stromatoporoids appear to have been capable in some cases of growing directly on the muddy substrate (see the project on stromatoporoid and coral substrates in this website).
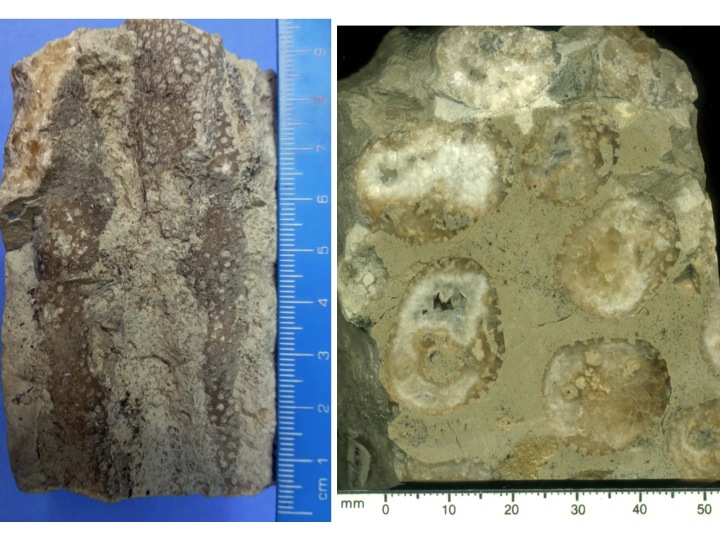
Figure 6B: A specimen of a Jurassic coral Thamnasteria from England, showing how an original aragonite mineralogy can be destroyed and replaced by calcite. The left photo shows the side of two branches of this fingerlike branching coral; the corallites are apparently well-preserved. However the right photo is a polished transverse section, showing that the coral internal structure is completely lost, and replaced by coarse crystalline calcite. The coral aragonite was dissolved once the micritic sediment around the branches had been lithified, leaving an external mould of the fossil. Later the empty fingerlike holes were filled with calcite cement, preserving the surface shape of the coral, but not its internal structure. Some of the holes were not completely filled with calcite, leaving crystal terminations in cavities. Thus it is clear that this coral was made of a form of calcium carbonate that was easily dissolved, that is, aragonite; stromatoporoids, in contrast, do not show such dissolution and replacement, and this makes it difficult to accept stromatoporoids as being aragonite originally.
The differences between stromatoporoids and other fossils are also reported by Rush and Chafetz (1991) and Smosna (1984) in Devonian stromatoporoids, from New York and Virginia respectively, and in personal communication from Carl Stock (2013) on unpublished observations from the Pridoli (latest Silurian) of New York. This consistent difference between stromatoporoids and aragonitic mollusc shells (and the Jurassic coral shown above in Fig. 6B) creates a significant problem for interpretations that stromatoporoids were originally aragonitic. For reference, note that the two modern calcified sponges most similar to stromatoporoids, Calcifibrispongia and Astrosclera, both have aragonite calcareous skeletons (Stearn, 2010c).
3. CROSSED POLARISED LIGHT (XPL) APPEARANCE
|
As long as the thin section is thin enough, XPL provides greater clarity of the appearance of the stromatoporoid diagenetic structure than the Plane Polarised Light (PPL) view, because the crystals are seen in various positions of extinction providing a greater contrast between them. Note, however, that it is essential to make thin sections thin enough, because the high birefringence of calcite makes the PPL and XPL views almost exactly the same in thick thin sections. Also, stromatoporoid taxonomy is undertaken in PPL because it gives more intense light and does not have the interrupting effect, of XPL, of obscuring the skeletal structure by extinction of the crystals that are in different orientations in different parts of the same thin section. Thus it is perhaps not surprising that the recrystallised structure of stromatoporoids is not widely known, simply because it is not visible in thin sections usually used for taxonomy.
Furthermore, the PPL & XPL study in normal light can be supplemented by the use of cathodoluminescence (CL) imaging, shown in this webpage. Note that normal light (PPL & XPL) gives a different view from CL and there is no primary relationship between them; the cements viewed in XPL overprint the fabrics seen in CL. CL thus gives the appearance of the stromatoporoid structure at an earlier stage it its diagenesis, so the fabrics seen in XPL are interpreted as later diagenetic change in the stromatoporoid.
The recrystallization of stromatoporoid skeletons exhibits a feature that is probably unique in fossils. In XPL, vertical sections of the skeleton of almost all species shows an arrangement of irregular elongated calcite crystals orientated normal to the growth surface, crossing the lamination, thereby cutting both the stromatoporoid gallery cements and skeleton alike. Fig. 7 shows the edge of a fragment of stromatoporoid in grainstone composed of several fossil groups, but the crystal structure of the stromatoporoid stops abruptly at the stromatoporoid margin; these bioclasts are fragments of shelly organisms that grew in environments and formed part of a sequence of stromatoporoid-bearing limestone, so were deposited all together, yet the diagenesis within the stromatoporoid affected only the stromatoporoid. This observation, that stromatoporoids have irregular elongated crystals in contrast to other fossils, is repeated by the author in different facies of Silurian and Devonian stromatoporoids. In fact every single stromatoporoid that I have studied in thin section shows this irregular bladed structure, regardless of taxon, of environment or of geological age. Even very different skeletal architectures (therefore very different taxa) show the same recrystallised fabric. Fig. 7 particularly shows the contrast with crinoids, since a] crinoids do not show such irregular crystals and b] stromatoporoids lack syntaxial cements.

Figure 7: Fragment of an unidentified stromatoporoid in vertical section (lower half of photo) and crinoidal-shelly grainstone above, from Ludlow of Gotland. A) plane-polarised light (PPL); B) cross-polarised light (XPL). B shows the characteristic irregular elongated calcite crystals typical of stromatoporoids, with the crystals cross-cutting the stromatoporoid skeleton, but terminating sharply at the stromatoporoid margin, demonstrating that the internal diagenetic alteration is limited to the stromatoporoid.
Fig. 8 shows apparently well-preserved Habrostroma from Silurian of New York (sample provided by Carl Stock), demonstrating that the irregular crystals, which in transverse section are approximately equant.

Figure 8: Stromatoporoid (Habrostroma) from the Upper Silurian of New York. A and B- PPL and XPL views in vertical section showing the irregular elongate calcite crystals in B, typical of stromatoporoids. C and D- enlargments of A and B, respectively, showing the detail of relationship between irregular crystals in XPL and the stromatoporoid structure. E and F- transverse section of the same specimen, showing the irregular crystals are approximately equant in transverse view. Thin sections were provided by Carl Stock, with thanks (I mean, I thank him!!)
Figs. 9 and 10 show two further taxa (Eostromatopora impexa and Densastroma pexisum) from the Wenlock of Gotland (Sweden), showing the same irregular calcite cement cross-cutting the stromatoporoid structure, irrespective of stromatoporoid taxa (see also Fig. 12). This characteristic irregular cement is so pervasive that even badly recrystallized stromatoporoids can be recognized as stromatoporoids in cross-polarized light, including cases where any skeleton-based taxonomic features are further altered beyond recognition.

Figure 9: Vertical sections of Eostromatopora impexa from the Visby Formation, Wenlock of Gotland in both PPL and XPL. In C, the gallery space is shown by small equant areas of clear calcite cement left and right. Photos C and D emphasise that the irregular calcite crystals in XPL cross-cut the stromatoporoid skeleton and continue into the gallery space, demonstrating: 1] that this skeleton is altered, regardless of its apparently well-preserved structure; and 2] that the gallery space was filled with cement that was then altered by diagenesis in continuity with the stromatoporoid skeleton. Compare this figure with Fig. 10.

Figure 10: Low, medium and high power views of vertical sections of Densastroma pexisum from the Visby Formation, Wenlock of Gotland in both PPL and XPL. Compare this figure with Fig. 9, they are very different stromatoporoid skeletal structures (taxa) in the same environment but with the same style of alteration. Examples in Figs. 7 and 8 are from two more different taxa and settings, so that all four stromatoporoid taxa (Densastroma, Habrostroma, Eostromatopora and the unidentified taxon in Fig 7, which is not one of the other three) show the same characteristic irregular elongated structure, visible also in Figs. 3 and 6 in PPL. See text for discussion.
The diagenetic character of stromatoporoids illustrated in Figs. 7-10 is poorly reported in the literature, the only XPL illustrations that I am aware of are in Rush and Chafetz (1991) and Smosna (1984). The probable reason for its uncommon description is that stromatoporoid taxonomy normally uses thin sections of 50-80 microns thickness; nevertheless even in PPL the fabric is visible if the sections are thin enough (see also Figs. 3, 5 and some plates in Dong, 2001). Thus thin sections need to be the normal 30 micron thickness, or less, for easy observation of this fabric. Smosna (1984, p.1004) provided a concise description of the recrystallisation into irregular crystals that exactly matches the observations made in this webpage (and the paper you can download, see url at the top of this page) of such alteration in stromatoporoids from various ages and facies. Smosna (1984) also noted that the crystals cross stromatoporoid lamination in vertical section. Furthermore, Smosna (1984) observed that the crystals do not pass through areas of a stromatoporoid where sediment interrupted growth, further emphasising that the diagenetic change is restricted to the stromatoporoid. Finally, Smosna (1984) recorded undulose extinction in the crystals, which can also be appreciated in Figs. 8-10.
The process of stromatoporoid diagenesis took place not just in the stromatoporoid skeleton, but also in the gallery-filling cement. Fig. 13 below shows that CL reveals the sequence of cements in galleries, which is overprinted by the elongated irregular crystals. The process of alteration remains unexplained and is an avenue for future investigation. Smosna (1984) interpreted the irregular calcite as having formed by inversion to calcite from the original mineralogy in freshwater environments; this may or may not apply in all cases, particularly in view of the CL evidence of later burial cement in the particular samples shown in Figs. 11-14 and 17 below, but is certainly possible in other cases because of the relatively shallow water environment of stromatoporoids. The recrystallised structure survives further alteration of the skeletons in stromatoporoids that can be identified, but some specimens are further altered so the skeleton itself is no longer recognisable (no photos included in this webpage yet). However, a curious feature of these more heavily altered examples is that the bladed structure can still be seen in XPL, and allows the investigator to identify that even an altered grain is a stromatoporoid despite its taxonomic identification being impossible to determine.
In summary, because of the differences between stromatoporoids and brachiopods, molluscs and crinoids with which they occur, the issue of the original mineralogy of stromatoporoids remains a problem unlikely to be resolved by light microscopy. More data are required.
4. CATHODOLUMINESCENCE (CL) APPEARANCE
|
Stromatoporoids occur very commonly with crinoids (presumed originally Hi-Mg calcite, HMC) and both show a prominent speckled appearance in CL, which, in stromatoporoids, is sharp-bounded against the gallery-filling calcite cement. The CL view may show the original fabric of the stromatoporoid, but this depends on the interpretation of CL features, discussed by Kershaw (1994). However, crinoids have large overgrowths of non-luminescent cement, reflecting their single-crystal composition, in contrast to the small non-luminescent first generation cement on stromatoporoid fragments. These features are shown in the following Figs. 11 to 14.

Figure 11: Cathodoluminescence (CL) of crinoid debris from a stromatoporoid biostrome, Ludlow of Gotland. The left photo in PPL shows the granular appearance of the crinoid fragments deposited as a grainstone (no sediment in between the grains); the space was filled with cement. The right photo in CL shows the sequence of events was more complex. The non-luminescent cement formed syntaxial growths on the crinoid, and developed large overgrowths; this was probably in the shallow burial aerobic zone because the lack of luminescence is generally interpreted as lack of Mn. Thus there would also be no ferrous iron present to quench any luminescence because both ferrous iron and Mn are present dissolved in the porewaters in only low oxygen conditions. The succeeding bright cement is likely to represent shallow low oxygen burial, where ferrous iron was removed from the system by sulphide ions released from the seawater sulphate by sulphate-reducing bacteria, and deposited as pyrite. The last, dull zone, is likely indicating a mixture of luminescent Mn and luminescence-quenching ferrous Fe, in deeper burial. A key aspect is the speckled appearance of the crinoids.

Figure 12: Enlargement of part of Figure 12, to emphasise the cement sequence in CL and the speckled appearance of the stromatoporoids.

Figure 13: plane light and CL of a stromatoporoid (Simplexodictyon yavorskyi), showing the speckled appearance of the stromatoporoid skeleton.

Figure 14: A set of photographs comparing the speckled appearance in CL of crinoids (left photos) and stromatoporoid (right photos), collected from the same stromatoporoid biostrome, middle Ludlow, Silurian, Gotland, Sweden. Whether the similarity between crinoids and stromatoporoids in CL indicates a similar original mineralogy or not is open to debate. In the CL photographs, because both samples come from the same bed they show the same sequence of cements, from non-luminescent, through bright to dull, indicating the same diagenetic environment evolution influenced both samples.
The similar speckled appearance is circumstantial evidence of similarity between the two fossils’ mineralogy, but CL is not a reliable guide to mineralogy of carbonates and the similarity may be coincidental. Nevertheless, Rush and Chafetz (1991) demonstrated dolomitic microcrystals formed by diagenesis within stromatoporoid skeletons from the Devonian of New York, inferring that the stromatoporoids were HiMg calcite. The CL images in Fig. 13 & 14 are interpreted here as evidence of the original relationship between the stromatoporoid skeleton and the gallery cement, such that the galleries were most probably infilled with cement after soft tissue decayed and was replaced by water in the galleries. The cements show a sequence of evolution from non-luminescent (probably oxygenated water in shallow burial), through bright luminescence (probably shallow anoxic position just below the redox boundary), to dull luminescent (probably deeper burial). See also Scoffin (1987) for further discussion of environments of different CL phases.
5. APPEARANCE OF STROMATOPOROIDS AND SYMBIOTIC CORALS
|
Stromatoporoids frequently contain symbiotic organisms, tabulate and rugose corals being common. Coral tubes intergrew with the stromatoporoid host, and because individual coral tubes run for many centimetres vertically through a single stromatoporoid, a logical interpretation is that the host and coral grew at similar rates. It may be that the corals modified their growth rates to match the stromatoporoid. The following photos show thin sections of one taxon of stromatoporoid (Petridiostroma convictum), with two corals, a rugosan (Tryplasma) and a syringoporid tabulate.

Figure 15: Intergrown corals inside a stromatoporoid, Petridiostroma convictum, from the Ludlow of Gotland. Photos A) and B): Vertical and tangential thin sections of different samples showing the close intergrowth of corals and stromatoproid. Photo A has syringoporid coral tubes that developed as the stromatoporoid grew, and their growth rates were presumably well-matched; Photo B has both syringoporid (small circles) and branching rugose corals. Photos A and B are negative photographs. Photos C) and D): XPL views of very thin section (15 microns) of detail of structure of Petridiostroma convictum; the stromatoporoid skeleton is visible as a dusty appearance on the calcite cement, demonstrating pervasive alteration of the stromatoporoid, even though its taxon is clearly identifiable at more normal thickness in Photos A and B. E) and F): XPL views of very thin sections of Petridiostroma convictum stromatoporoid with rugose coral (E) and syringoporid (F). In Photo E, only the rugosan is visible, but shows its very well preserved wall structure; in F, the syringoporid wall structure is partly altered, but is better preserved than the stromatoporoid “dusty” fabric in Photos C and D. Photos A-F therefore demonstrate not only the intimate relationship between corals and stromatoporoids in intergrowth, but also the differences in preservation.

Figure 16: Enlargement of Fig. 15F, showing an XPL view of a syringoporid tube to emphasise details of the laminated coral wall in an altered stromatoporoid.

Figure 17: PPL and CL views of inter grown Petridiostroma convictum and syringoporid, showing the speckled appearance of the stromatoporoid skeleton compared to the more even-coloured coral walls. This difference may be due to the better preservation of the coral, but these photos emphasise that the interpretation of CL images must be made with caution, since the textural differences between the coral and the stromatoporoid in this case are not great. Nevertheless, it is clear from the photos in this section that the corals are better preserved than the stromatoporoid, and demonstrate that it is likely that the biology of the once-living organisms created different minerals in creatures living together in the same environment.
6. SCANNING ELECTRON MICROSCOPE (SEM) STUDY
|
I do not have any SEM images of the samples of stromatoporoids used in this study. However, SEM images of stromatoporoids have been used by other authors to investigate the structure of the stromatoporoid skeleton (Rush and Chafetz, 1991; Smosna, 1984; Stearn, 1977, 1989a; Stearn and Mah, 1987). In each case, polished surfaces were etched and examined with secondary electrons, illustrations being of the microtopography of etched surfaces. In each case the stromatoporoid skeleton is revealed as having a sharp contact with the surrounding gallery cement, as can be seen in thin sections in PPL. There is no description in the above references of crystal boundaries passing from the skeleton into the cement, identified in XPL in Figs. 7-10. However, careful examination of published SEM photographs in those references above shows curving and irregular lines in the structure subject to greater etching (e.g. Rush and Chafetz, 1991, Fig. 3; Stearn 1977, Stearn 1989a, Fig. 1B; Stearn and Mah, 1987, Fig. 1C,E,F). Such lines, also mentioned by Stearn (1977), may be interpreted as boundaries of the large irregular crystals that cross-cut the stromatoporoid skeleton and overprint the smaller crystals making up the skeleton itself. Thus the visual evidence from published SEM photographs may be considered as being compatible with the diagenetic feature recognisable in XPL that overprints the stromatoporoid skeleton. Please take a look at those published papers to verify my comments here.
7. IMPLICATIONS FOR PALAEOECOLOGY AND PALAEOGEOGRAPHY
|
Cherns and Wright (2000) demonstrated that loss of aragonite fossils by dissolution in molluscs is a common feature of the rock record. However, this observation forms a sharp contrast to a key Silurian example, on the island of Gotland, where originally-aragonitic mollusc shells show exceptional preservation in silica, which is resistant to dissolution. Thus the shells were converted from aragonite to silica early in their diagenesis and this preserved the original community structure of the organisms in that deposit. Their work illustrates the under-representation of these organisms in the fossil record due to diagenetic dissolution. However, stromatoporoids occurring together with dissolved molluscs, as illustrated in Figs. 5 and 6A, do not exhibit dissolution loss, so this shows that stromatoporoid taxonomic assemblages are not under-represented, and that all taxa of stromatoporoids are likely to be equally represented in the fossil record. This key point provides confidence for palaeoecological and palaeogeographic studies on stromatoporoids. Thus the reconstructions of biogeographic distributions of stromatoporoids, such as those provided by Stock (1990) for the Devonian, are robust within the limitations of the accuracy of continental positioning and completeness of stromatoporoid collections.
8. WIDER IMPLICATIONS FOR THE CHEMISTRY OF THE OCEANS
|
The thriving debate in the literature about changing seawater chemistry through time led to the concept of calcite and aragonite seas, widely published. The Palaeozoic stromatoporoids lived in a time regarded as being calcite seas, and some compilations include stromatoporoids as calcite fossils. The problem, shown in the illustrations above, demonstrates that the original mineralogy of stromatoporoids cannot be unequivocally stated as being either calcite or aragonite. Note that stromatoporoids are always altered, yet the alteration does not normally prevent identification using skeleton-based taxonomy.
Therefore it would be very sensible for researchers to NOT attribute an original mineralogy to stromatoporoids in any compilation of taxa for assessment of calcite and aragonite seas.
9. REFERENCES
|
The reference list below is the full list from Kershaw, S. 2013. Palaeozoic stromatoporoid futures: a discussion of their taxonomy, mineralogy and applications in palaeoecology and palaeoenvironmental analysis. Journal of Palaeogeography, 2, 163-182. This paper is available on open access from the following link: http://www.journalofpalaeogeography.org/EN/volumn/volumn_1138.shtml. Note that only some of the references below are cited in the text of this part of the webpage, but I gave you the full list in case you are interested to read other papers as well.
Cherns, L. and Wright, V.P. 2000. Missing molluscs as evidence of large scale, early skeletal aragonite dissolution in a Silurian sea. Geology, 28, 791-794.
Da Silva, A-C, Kershaw, S. and Boulvain, F. 2011a. Stromatoporoid palaeoecology in the Frasnian (Upper Devonian) Belgian platform, and its applications in interpretation of carbonate platform of carbonate platform environments. Palaeontology, 54, 883-905.
Da Silva, A-C, Kershaw, S. and Boulvain, F. 2011b. Sedimentology and stromatoporoid palaeoecology of Frasnian (Upper Devonian) carbonate mounds in southern Belgium. Lethaia, 44, 255-274.
Da Silva, A.C., Kershaw, S., Boulvain, F. and Reitner, J. 2011c Long-expected! - First record of demosponge-type spicules in a Devonian stromatoporoid (Frasnian, Belgium). In Aretz, M., Delculée, S., Denayer, J. and Poty, E. (eds). 11th Symposium on Fossil Cnidaria and Sponges, Liège, August 19-29, 2011, Abstracts. Kölner Forum Geol. Paläont., 19, 32-33.
Dong, Deyuan 2001. Stromatoporoids of China. Science Press, Beijing, 423 pages (Chinese with English abstract).
Gao Jianguo and Copper, P. 1997. Growth rates of Middle Paleozoic corals and sponges: Early Silurian of eastern Canada. Proceedings of the Eighth International coral Reef Symposium, 2, 1651-1656.
Goldfuss, A. 1826. Petrefacta Germaniae. 1st Ed. Verlag von List and Francke, Dusseldorf, 761 pp.
Kazmierczak, J. 1976. Cyanophycaean nature of stromatoporoids. Nature, 264, 49-51.
Kershaw, S. 1981. Stromatoporoid growth form and taxonomy in a Silurian biostrome, Gotland. Journal of Paleontology 55, 1284 - 1295.
Kershaw, S. 1984. Patterns of stromatoporoid growth in level-bottom environments. Palaeontology, 27, 113 - 130.
Kershaw, S. 1987. Stromatoporoid - coral intergrowths in a Silurian biostrome. Lethaia, 20, 371 – 382.
Kershaw, S. 1990. Stromatoporoid palaeobiology and taphonomy in a Siluran biostrome, Gotland, Sweden. Palaeontology 33: (3), 681 - 705.
Kershaw, S. 1994. Cathodoluminescence of Silurian stromatoporoids from Gotland, Sweden. Courier Forschungsinstitut Senckenberg, 172, 307-318.
Kershaw, S. 1997. Palaeoenvironmental change in Silurian stromatoporoid reefs, Gotland, Sweden. Bolletin Real Sociedad Española de Historia Natural (Section Geologicas), 91 (1-4), 331-344.
Kershaw, S. 1998. The Applications of stromatoporoid palaeobiology in palaeoenvironment analysis. Palaeontology, 41, 509-544.
Kershaw, S. 2012. Paleoecology. Part E, volume 4, Chapter 13, Hypercalcified Porifera. Lawrence Press, University of Kansas, Treatise Online, 31, 1-24.
Kershaw, S. and Brunton, F. 1999. Palaeozoic stromatoporoid taphonomy: ecologic and environmental significance. Palaeogeography, Palaeoclimatology, Palaeoecology, 147, 1-16.
Kershaw, S., Wood, R. and Guo, L. 2006. Stromatoporoid response to muddy substrates in Silurian limestones. GFF, 128, 131-138.
Reitner, J. and Engeser, T.S. 1987. Skeletal structures and habitats of Recent and fossil Acanthochaetetes (subclass Tetractinomorpha, Demospongiae, Porifera). Coral Reefs, 6, 13-18.
Rush, P.F. and Chafetz, H.S. 1991. Skeletal mineralogy of Devonian stromatoporoids. Journal of Sedimentary Petrology, 61, 364–369.
Sandström, S. and Kershaw, S. 2008. Palaeobiology, ecology, and distribution of stromatoporoid faunas in biostromes of the mid-Ludlow of Gotland, Sweden. Acta Palaeontological Polonica, 53, 293-302.
Scoffin, T.P. 1987. An introduction to carbonate sediments and rocks. Blackie, Glasgow & London, 274pp.
Smosna, R. 1984. Diagenesis of a stromatoporoid patch reef. Journal of Sedimentary Petrology, 54, 1000-1011.
Stearn, C. W. 1977. Studies of stromatoporoids by scanning electron microscopy. Burreau de Recherches Géologiques et Minières, Mémoir, 89, 33–40.
Stearn, C.W. 1983. Stromatoporoids from the Blue Fiord Formation (Lower Devonian) of Ellesmere Island, Arctic Canada. Journal of Paleontology, 57, 539-559.
Stearn, C. W. 1989a. Specks in the microstructure of Paleozoic stromatoporoids. Proceedings of 5th International International Symposium of Fossil Cnidaria, Brisbane. Association of Australasian Palaeontologists Memoir, 8, 143–148.
Stearn, C.W. 1989b. Intraspecific variability and species concepts in Palaeozoic stromatoporoids. Association of Australasian Palaeontologists Memoir, 8, 45-50.
Stearn, C.W. 2010a. Morphological affinities of Paleozoic Stromatoporoidea to other fossil and Recent groups. Part E, volume 4, Chapter 9E, Hypercalcified Porifera. Lawrence Press, University of Kansas, Treatise Online, 7, 1-9.
Stearn, C.W. 2010b. Paleozoic Stromatoporoidea: general introduction. Part E, volume 4, Chapter 9A, Hypercalcified Porifera. Lawrence Press, University of Kansas, Treatise Online, 5, 1-3.
Stearn, C.W. 2010c. Microstructure and mineralogy of Paleozoic stromatoporoids. Part E, volume 4, Chapter 9D, Hypercalcified Porifera. Lawrence Press, University of Kansas, Treatise Online, 6, 1-25.
Stearn, C.W. 2011. Internal morphology of the Paleozoic Stromatoporoidea. Part E, volume 4, Chapter 9C, Hypercalcified Porifera. Lawrence Press, University of Kansas, Treatise Online, 18, 1-37.
Stearn, C. W., & A. J. Mah. 1987. Skeletal microstructure of Paleozoic stromatoporoids and its mineralogical implications. Palaios, 2, 76–84.
Stock, C.W. 1982. Upper Devonian (Frasnian) Stromatoporoidea of north-central Iowa: Mason City Member of the Shell Rock Formation. Journal of Paleontology, 56, 654-679.
Stock, C.W. 1990. Biogeography of the Devonian stromatoporoids. In: McKerrow, W.S. and Scotese, C.R. (eds). Palaeozoic Palaeogeography and Biogeography. Geological Society Memoir, 12, 257-265.
Tourneur, F., Lachkhem, H. and Mistiaen, B. 1994. Trypanopora conili nov. sp. (Annelida?) from the Couvin Limestone, Eifelian of the southern margin of the Dinant Synclinorium (Belgium). Biological affinities and relationships with its hosts. Mémoires Institut Géologique de l’Université Catholique de Louvain, 35, 83-122.
Vacelet, J. 1985. Coralline sponges and the evolution of Porifera. Special Publication of the Systematics Association, 28, 1-13.
Webby, B.D. and Kershaw, S. 2011. External morphology: shapes and growth habits. Part E, volume 4, Chapter 9, Hypercalcified Porifera. Lawrence Press, University of Kansas, Treatise Online, 25, 1–73.
Webby, B.D. and Zhen Yong-Yi. 1993. Lower Devonian stromatoporoids from the Jesse Limestone of the Limekilns area, New South Wales. Alcheringa, 17, 327-352.
Wood, R., Reitner, J. and West, R. R. 1989. Systematics and phylogenetic implications of the haploscerid stromatoporoid Newellia mira nov. gen. Lethaia, 22, 85-93.
Young, G. and Kershaw, S. 2005. Classification and controls of internal banding in Palaeozoic stromatoporoids and colonial corals. Palaeontology, 48, 623-651.
Zapalski, M.K. and Hubert, B.L.M. 2010. First fossil record of parasitism in Devonian calcareous sponges (stromatoporoids). Parasitology, 138, 132-138.
Zhen, Yong-Yi and West, R.R. 1997. Symbionts in a stromatoporoid-chaetetid association from the Middle Devonian Burdekin Basin, north Queensland. Alcheringa, 21, 271-280.
Click here to return to the GEOSCIENCE RESEARCH PROJECTS header page
Click here to return to the HOMEPAGE
| | | | | | | | |


















